Contact Us
We are committed to providing quality services and products for our customers. Please reach out to us with any questions you may have and a member of our team will get in touch with you as soon as possible. Please complete the contact us form.
How to Order
How do I place an order with a quote?
If you are ordering from our product catalog, simply add the desired item(s) to your cart and place an online order.
If you will be ordering a customized product or any of our services, please complete the request a quote form first. Once you have a quote, submit your order using one of these two methods:
- Purchase Order: Email or fax your purchase order.
- Credit Card: Email or fax your signed PO specifying that you will pay with a credit card. DO NOT send credit card information via e-mail. Upon the order completion, you will receive an invoice with details on how to pay with a credit card.
Email: [email protected]
Phone: +1-301-822-4513
Fax: +1(301)251-6110
How should I send my plasmid?
Plasmids in TE buffer may be shipped at room temperature. Please check with us about shipping options if you use other buffers. We usually recommend shipping on dry ice (or similar packaging) to reduce the chance of DNA degradation. Plasmids sent on filter paper will not be accepted. We also strongly recommend overnight shipping using an express carrier (e.g., UPS, FedEx) with tracking to minimize transit time.
To ensure your plasmids arrive at our safely, we recommend the following:
1. Always wrap plasmids in parafilm or use screw tops in order to ensure the vial stays closed during transit.
2. Place your vials inside a hard outer container or use plenty of bubble wrap to ensure the plasmids are adequately protected during transit.
3. If you need to send your plasmids on dry ice then try to use dry ice pellets instead of large slabs which helps keep the vials more insulated and distributes weight better than large chunks of dry ice.
How should I address my package?
Charles River Laboratories
Attn: LOGISTICS
Ref # [quote number]
5 Research Court
Rockville, MD 20850 USA
What should I include in my package?
All packages need to include a copy of the corresponding quote for the plasmids included in the package. The number of plasmids included in the package should also be noted to minimize the possibility of missing a plasmid within a package.
Not including a copy of the quote inside the package increases the likelihood of a plasmid being incorrectly logged or matched to the wrong order which can cause significant order delays.
How do I know Charles River has received my DNA?
After your package has been delivered, your plasmid will be logged into our system and given an internal tracking number. A Plasmid Received confirmation email will be sent to the email provided on the quote and will indicate how many plasmids were received and to which quote they have been matched. If multiple plasmids for multiple orders were shipped in the same package then multiple Plasmid Received confirmation emails will be sent.
If you do not receive a Plasmid Received confirmation email within 48 hours of your package being delivered please contact us immediately.
How much plasmid do I need to send?
Please refer to the table below for the recommended amounts to send us for your requested service. When possible, we recommend sending DNA obtained from a maxiprep. If you do not have the recommended amount of plasmid, we can prepare endotoxin-free DNA from your sample for an additional fee.
| Service | Amount of plasmid to send |
| Cloning | 5-10 µg at conc. of >0.5 µg/µL |
| Transformation (plasmid preps) | >1 µg |
| Small-scale AAV packaging Titer: 5x1012 GC/mL |
>150 µg |
| Large-scale AAV packaging Titer: 1013 GC/mL |
>300 µg |
| Small-scale lentivirus packaging Titer: 2x108 IFU/mL |
>150 µg at 1 µg/µL or higher |
| Large-scale lentivirus packaging Titer: 109 IFU/mL |
>200 µg at 1 µg/µL or higher |
| Adenovirus packaging | 5-10 µg at conc. of >0.5 µg/µL |
When and how will my order be shipped?
If the requested product is in stock, your order will be processed and shipped as soon as possible. If your order is not available for immediate shipping (i.e., when ordering custom products and services), you will receive an estimated timeline on the quote you receive from our technical support team.
Orders containing viral particles will be shipped on dry ice. For domestic orders within the United States, we use FedEx “Priority Overnight” shipping. For countries outside of the US, we use FedEx “International Priority” shipping or World Courier. If you prefer a different shipping option, such as using your own courier account with FedEx or using your own courier, please let us know and we will only charge for the cost of dry ice and packaging. For international orders, you or your organization will be responsible for all import duties, taxes and brokerage fees that may be incurred from the shipment. To avoid these fees and to save cost on shipping, we recommend placing your order through one of our distributors.
How long does shipping take?
If your country/region is not listed below, please send us an email
| Region | Time |
|---|---|
| Continental US | Next day |
| Canada | Next day-4 days |
| Continental Europe | 2-5 days |
| Asia | 4-7 days |
| Australia/New Zealand | 4-7 days |
| South America | 5-7 days |
Frequently Asked Questions
Want to know more about any of our products or services? Start by checking out our Research-grade and CGMP FAQs. If you still have any questions, please don’t hesitate to contact us!
Research-grade FAQs
Which virus will work best for my experiments?
Below is a quick comparison of the three most popular recombinant viruses being used for research and gene therapy applications.
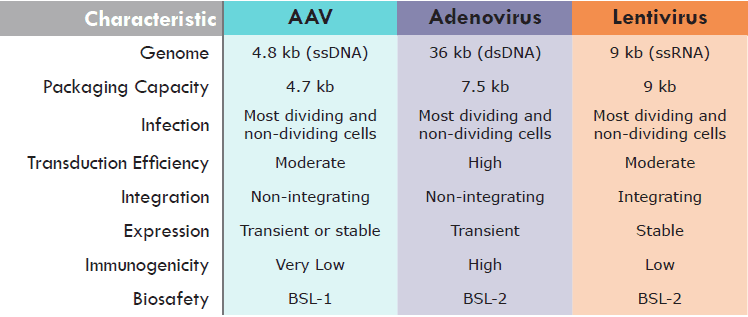
How do I know which scale of viral packaging to select?
What are the biosafety requirements for using AAV, adenovirus, and lentivirus?
Do you sell wild-type viruses?
How should viruses be stored?
Avoid repeated freeze-thaw cycles that can decrease the viral titer. Aliquot the viral stock upon arrival and keep the aliquots at -80°C for long-term storage.
For lentivirus:
Freeze-thaw cycles decrease lentivirus titer dramatically. Store the viral stock immediately upon arrival. Aliquot to desired vials right before use.
How does ordering and shipping work?
CGMP FAQs
How does a CGMP project differ from a research project?
What are the biggest contributors to the cost and timescale of a CGMP campaign?
What are the commonly overlooked components of a CGMP project?
What is the typical process for getting a quote?
- The number and types of specific vectors needed, plus the transgene sizes for each
- The scale of the project (total virus yield)
- What technology (materials or processes), if any, is available to be transferred to us
- Whether previous batches have been produced, and if those records are available for review
- Whether the formulation requirements are known
- The time frame by which the clinical-grade material must be produced
- The country (or countries) in which the clinical trials will be held

